What is CO2 Extraction Method?
When you walk into a dispensary (or meet your dealer) and look at the flower that’s available for sale, you know where it came from.
Not literally, of course; you don’t know exactly where it was grown. But can be pretty sure it was harvested, dried, and cured with generally-accepted industry techniques.
When you walk into a dispensary and see an assortment of cannabis concentrates, you’re generally in the dark. What were they sourced from? How were they made? How clean and pure are they?
Not all legal cannabis products carry that information on their labels, but there’s one clue you can look for: the method by which the weed’s cannabinoids were extracted.
We have to warn you before continuing – there will be a little science to digest. But it’s the only way to understand CO2 extraction and the products it can help create.
How Weed Concentrates Are Produced
Dabbing has become a big deal in the weed world. Concentrates like shatter, wax, and budder are available in most well-stocked dispensaries.
But most people don’t know how they’re made. Let’s fix that.
What are Cannabis Concentrates?
Cannabis concentrates, as their name suggests, contain concentrated amounts of the most valuable components of the weed plant. Trichomes containing cannabinoids (like THC and CBD) and terpenes are extracted from the plant and then processed to concentrate them even further.
The resulting products are much more potent than the flower that’s traditionally smoked. Extracts and concentrates contain at least twice as much THC as raw cannabis, and they may have as much as 90-95% THC content [1]. The vapor produced when concentrates are heated delivers a powerful high, and they contribute a strong potency boost when added to smokeables.
You may be familiar with rosin, a form of concentrate that can be made at home by squeezing buds in a heated flat iron. You can also make concentrate for dabbing by soaking weed in high-proof alcohol like Everclear and letting the alcohol evaporate by heating the mixture.
Those homemade concentrates are fine for dabbing, but they won’t deliver the same experience as commercially-produced shatter, wax, or other forms of BHO oil.
Higher-quality concentrates aren’t easy to create, and they can’t be safely (or in many cases, legally) produced by end-users. That’s because specialized equipment and techniques are required. The first, critical part of the process involves the extraction of cannabinoids and terpenes from weed plants.
Extraction Methods
When the resin is extracted from cannabis plants, it’s done by a chemical process that requires help from a solvent. Three solvents are commonly used [2].
Alcohol
This process is similar to the DIY method we’ve briefly described, but it takes that method to a very different level.
Ethanol chilled to -40°C soaks weed in an extraction chamber, and the resulting liquid is then run through a centrifuge, filtered, and put into a rotary evaporation machine. As you’d guess, the final concentrate is purer and more potent than the stuff you can make in your kitchen.
You can purchase a “home version” of this equipment for a couple of thousand dollars if you’re serious about making concentrates.
Butane
The majority of producers use hydrocarbon solvents like butane, propane, or hexane. The term butane hemp oil, or BHO, stems from this extraction method.
Chilled butane gas is run through cannabis plants as a solvent, to extract the plants’ trichomes. The resulting vapor is liquefied, the liquid is heated in a vacuum oven to remove leftover butane, and ethanol is used to remove impurities or plant matter that may still be in the product.
Butane is highly flammable and explosive, so butane extraction should never be attempted at home [3]. And as previously mentioned, the right equipment and expertise are required.
The third extraction method involves carbon dioxide.
Producing Weed Concentrates with CO2 Extraction

Carbon dioxide can also be used as a solvent to extract cannabinoids and terpenes from the weed plant – and its use provides several benefits.
First, carbon dioxide is plentiful. It’s easily obtainable without having to purchase and store expensive solvents.
Second, CO2 is clean, safe to use (unlike solvents like butane), and non-toxic. There’s no potentially-hazardous solvent residue left in the finished product that has to be eliminated. (There’s carbon dioxide in soda, too).
Finally, producers love using CO2 because it’s versatile.
Carbon dioxide normally takes the form of a gas, but it becomes a liquid when exposed to high pressure and/or temperatures. And at a specific pressure and temperature levels (which don’t damage weed), it becomes a so-called “supercritical liquid,” filling available space like gas but with the higher density of a liquid. That makes it perfect for use in extraction.
When CO2 is in its supercritical state, it’s able to break down the chemical structures of molecular substances – exactly what’s needed to separate cannabinoids and terpenoids from cannabis. In fact, it’s able to separate THC or CBD (or other cannabinoids) from everything else in trichomes to create isolate products (only THC or only CBD).
Carbon dioxide extraction is nothing new. It’s been around for more than 40 years, used by the cosmetic industry to extract essential oils from plants and by the coffee industry to remove caffeine.
More recently, it’s been adopted by many cannabis producers. Supercritical CO2 extraction is a lengthier and more expensive process than alcohol or butane extraction, but it creates the cleanest, safest and highest-quality cannabis concentrates.
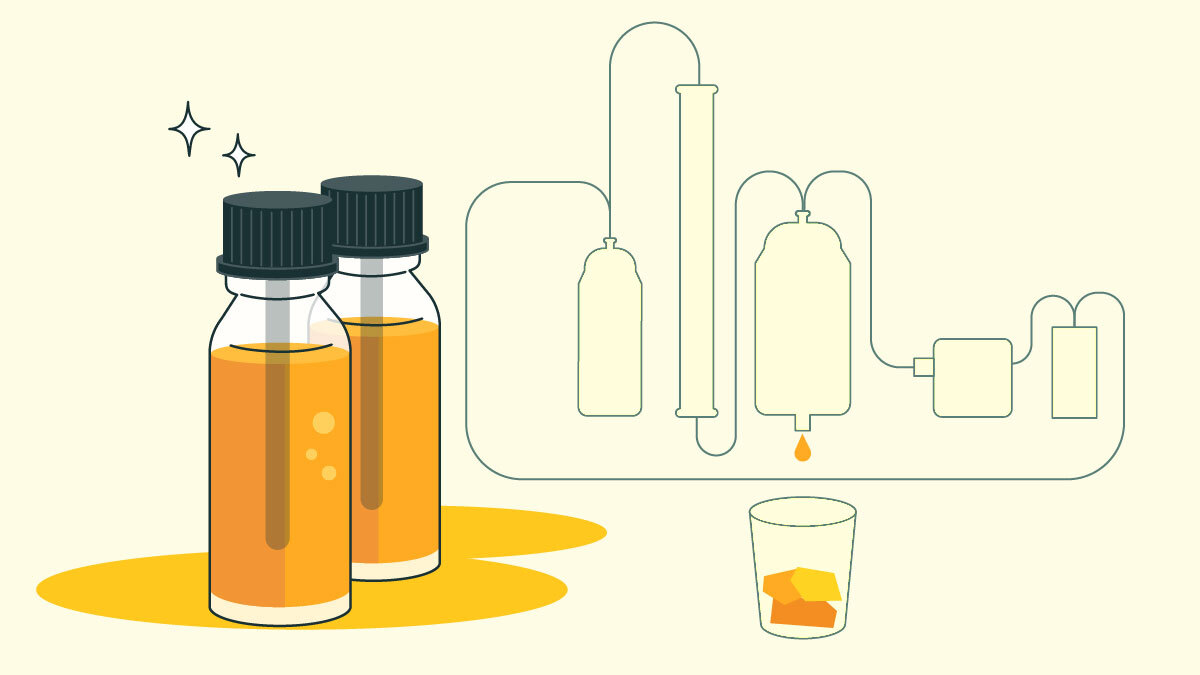
How CO2 Extraction Works
We’re not going into all of the technical details, because we don’t want to give readers a headache.
But here are the basics of how supercritical CO2 extraction is used to create weed concentrates [4].
- Special equipment changes CO2 from a gas to a liquid at temperatures around -70° and high pressure. It’s then reheated just enough to turn it supercritical.
- The supercritical CO2 is sent into a chamber containing cannabis, where it dissolves the membranes of the weed’s trichomes, and frees and captures its cannabinoids and terpenes.
- The solvent is then sent to a “condenser vessel” with lower temperatures and pressure. The carbon dioxide returns to gaseous form and is recaptured for later use. What’s left is a gooey cannabis substance that still contains some plant material.
- The product goes through “winterization” at cold temperatures, filtering, and distillation, which remove any excess material.
The supercritical CO2 extraction process allows technicians to remove specific cannabinoids and terpenes by using different pressure levels and temperatures. They can even reintegrate terpenes into the concentrate, if necessary, to improve its flavor and potency.
The final process creates concentrate with a liquid consistency which is ideal for vaping. The manipulation of extraction parameters also allows them to create different concentrates for vaping like shatter, wax, and crumble.
Bottom line? It’s not necessary to insist on concentrates that have been produced with CO2 extraction – but they’re likely to be the cleanest and most enjoyable dabbing product you can find.
CO2 Extraction: FAQ
Q: Does all of this mean that concentrates made with butane extraction, like BHO, aren’t safe?
A: Not at all. It just means that the ones made with supercritical CO2 extraction are safe for consumption by definition – as long as they’ve been produced by reputable companies and come with third-party COAs (certificates of analysis) showing that they don’t contain any harmful materials. Products extracted with butane are likely to be safe as well, as long as a reliable manufacturer has taken all the necessary steps to remove all traces of the solvent. Again, look for a COA before buying.
Q: Is CO2 extraction the same method I’ve seen advertised for CBD oil?
A: Absolutely. Just as it can be used to isolate THC in cannabis concentrates, it can be used to isolate cannabidiol for CBD products as well.